CHAPTER 3
MINERALS WITH RAINBOWS

Picture #58
Calcite Crystals (Calcium carbonate or CaCO3)
Coated with Marcasite (Iron di-sulphide or FeS2)
from Slovakia
Iron layers are the magic ingredients that coat many iridescent minerals.
The colors can be muted or vivid.
This European mineral specimen shows areas of varying colors, depending on the thickness of the iron coating.
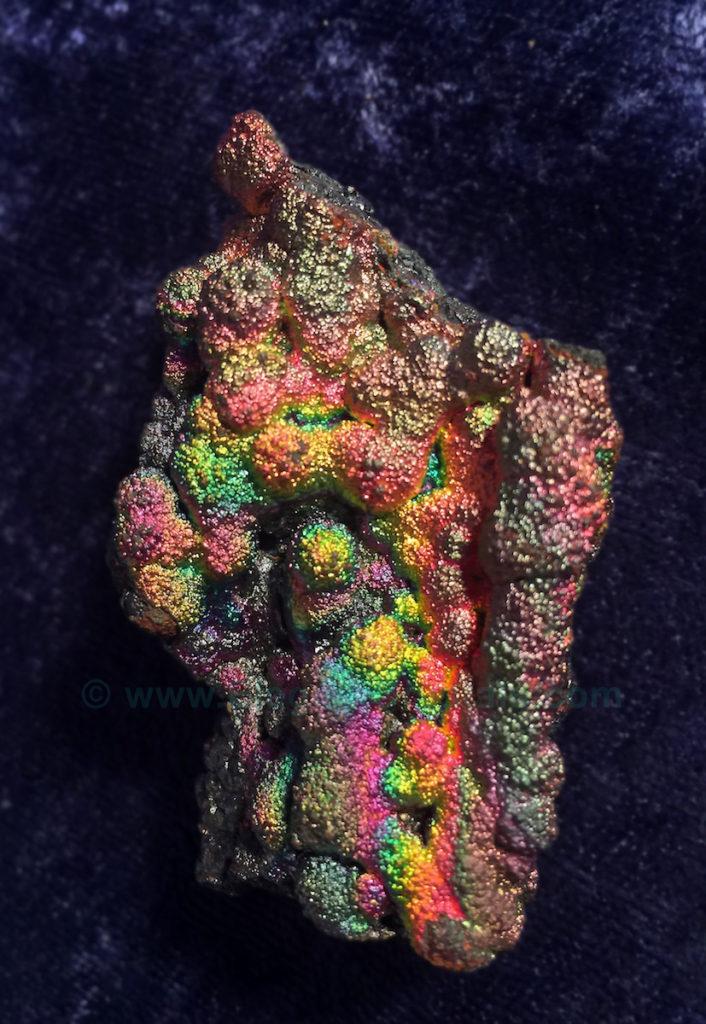
Picture #59
Goethite Crystals- Pronounced “GRRRR-tite”- FeO(OH)
These pure hydrated-iron crystals glow with unearthly rainbows.
The photograph only begins to show the intensity of the colors.
Crystallized iron occurs in several varieties of chemical compositions.
Even rust can crystallize!
It is then called hematite, which can form inside a quartz crystal or on its surface.
Rust is Fe2O3 + water
Hematite is Fe2O3

Picture #60
Amethyst on Chalcopyrite
SiO2 with CuFeS2
This tiny amethyst “castle,” one-inch-tall, is from Vera Cruz, Mexico.
It has a naturally flat base with hundreds of miniscule 1mm crystals around it.
It seemed a perfect fit when I balanced it on the chalcopyrite pedestal.
Chalcopyrite, a special kind of iron pyrite, has copper mixed into the formula.
Normally, chalcopyrite is a shiny gold color, just like regular pyrite.
Once the chalcopyrite is pickled in a mild acid bath, such as vinegar, the colors appear after the stone dries in the air.
This combination of iridescent colors is especially nice against the dark mineral background.
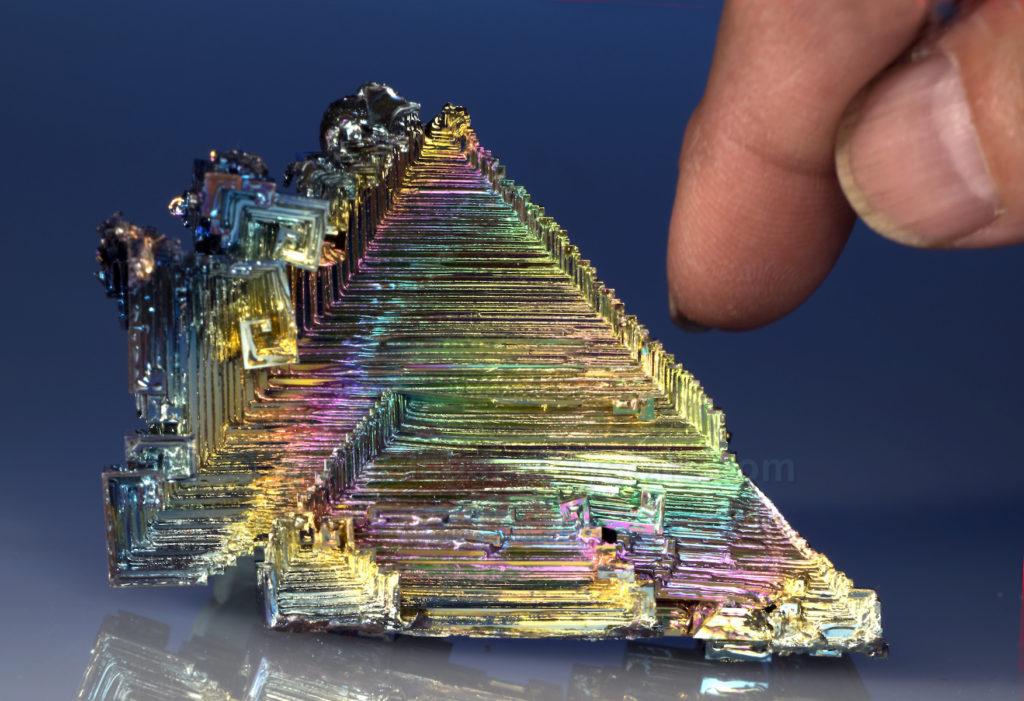
Picture #61
Man-made Bismuth Crystal
Bismuth, symbol Bi, is one of the few solid elements that can occur in an uncombined state in nature (free from being in a compound).
For that reason, it is called a native element.
It forms in the ground as small, typically 1-millimeter-wide crystals in bismuth ore.
When the ore is mined, crushed and refined, the resulting powdered bismuth metal can be highly purified.
The metallic dust is then melted again, in a special oven that has precise temperature controls.
When it reaches, 520.50 Fahrenheit, (2710 Celsius) the liquid metal is allowed to cool slowly, forming fantastic crystal shapes and colors.
The largest structures might be 3-inches-long, almost 80 times as large as the rare natural crystals.

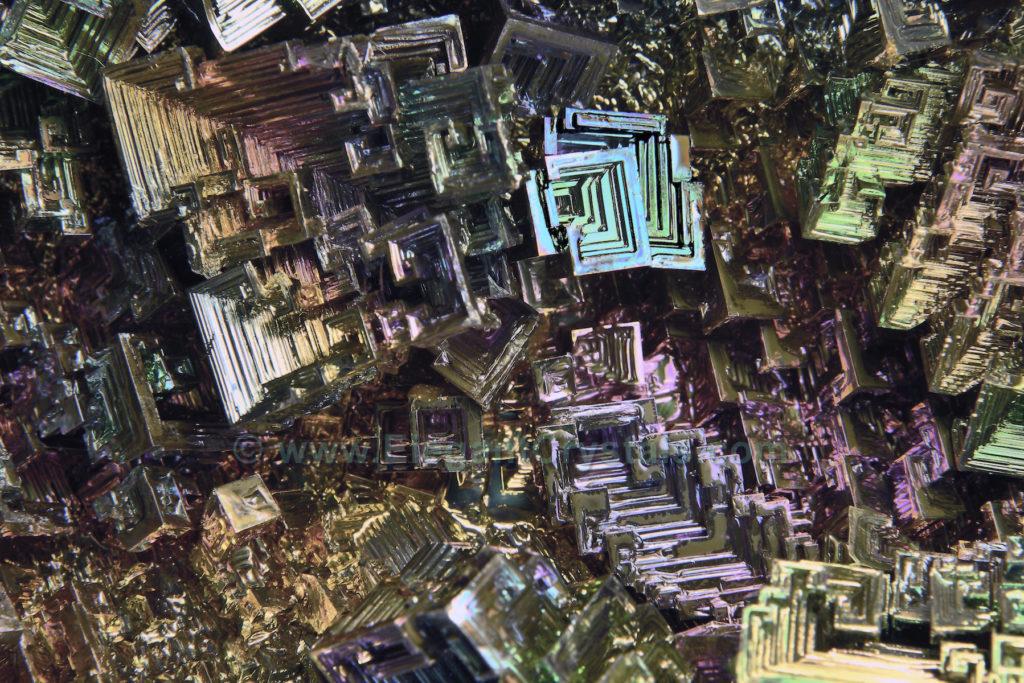
Picture #62
Man-made Bismuth Crystal
Pure bismuth metal shows a distinctive gray-white color with a reddish tinge.
How do you get it to flash rainbow colors?
After the bismuth powder is melted into a thick liquid, it crystallizes/hardens into these intricate patterns in the presence of air.
Oxygen combines with the outer layer of metal to form bismuth oxide, which shimmers and glows with true iridescence.
This peculiar shape reminds me of a viaduct, a pathway, and stairsteps.
It’s some kind of a rainbow bridge, drawing my eye to infinity and my heart to paradise.
I can imagine a temple doorway at the end of the path.
Will you enter this wonderful Crystal Realm with me?
The Crystal Palace
Poetry by Linda
There’s a place you can go
Where time stands still
And the answers are found within.
In the Crystal Palace
You can find a place
Where the Mystery never ends.
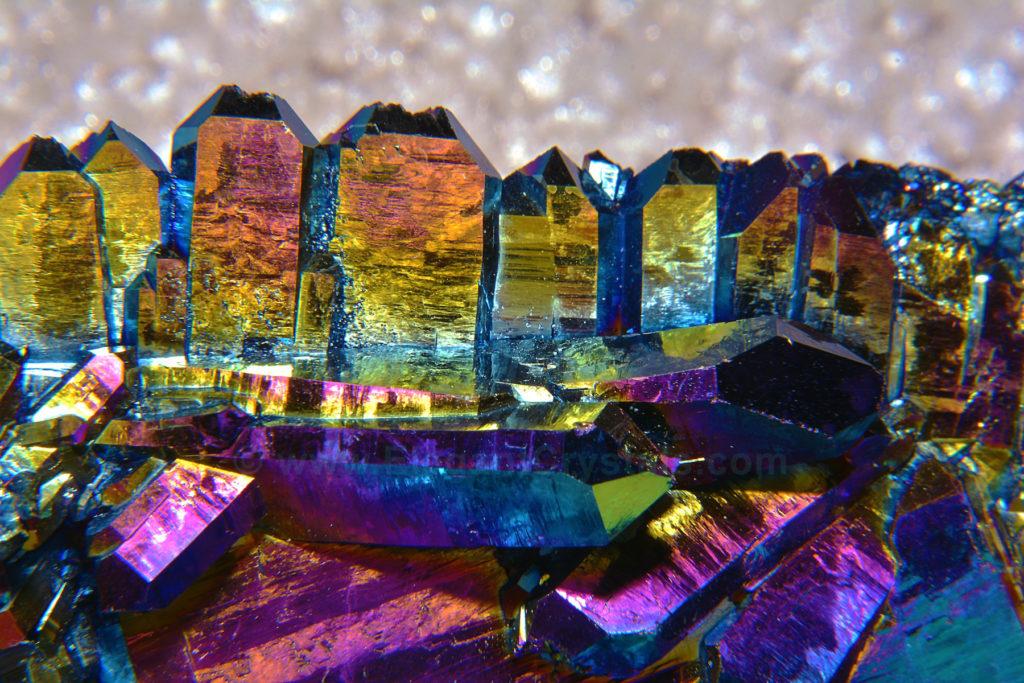
Picture #63
Man-made Bismuth Crystal Cluster/geode
In recent years, larger bismuth geodes are being grown in oversized “lapidary ovens.”
12-inch-tall geodes/clusters are now available, which contain hundreds of intricate crystals.
These groupings always seem interdimensional to me- doorways to new worlds of wonder.
Picture #64
Man-made Titanium Coating on Natural Arkansas Quartz Trade Name- Rainbow Aura Quartz
Natural crystals that iridesce are usually coated with a thin film of iron deposits.
In the laboratory, natural crystals, including many varieties of quartz, can be coated with various metals
to create shimmering color effects in different palettes.
The applied metal layers are only a few molecules thick, and may alternate with crystal-clear layers of added quartz.
This simulates natural iridescent growth displays, such as those found in labradorite and moonstone.
The metal coatings can be thicker or thinner layers of titanium, which affects the depth of the colors.
Denser layers give the beautiful, intense colors shown in the photo here.
Thin pure gold coatings yield blue tones that shift to purple as the stone is moved around.
Silver, platinum and other metals are sometimes used to create pastel iridescence on quartz.
These coatings are particularly lovely when applied to sparkly, tiny crystals called “druse” or drusy minerals.

Picture #65
4-inch-wide Calcite Crystal Ball
Calcium carbonate, CaCo3
Calcite is a form of natural crystal that occurs in many shapes, colors and places in the world.
Its most perfect expression is in clear crystals, which can be naturally faceted, or cut and polished, as in the picture here.
This crystal ball is nearly water clear and colorless, until light bounces off cleavage planes in the crystal.
Calcite is more fragile than most minerals, and needs to be handled gently.
The cleavage planes are areas where the calcite has weak atomic bonds that could split, if the crystal is dropped or bumped.
These atomic-level discontinuities create vivid rainbow colors where layers of crystal and vacuum spaces alternate.
Typically, the cleavages are parallel to each other, giving the impression of rainbow layers stacked on top of one another.
These parallel rainbows are much more common in calcite than in quartz.
The interactions of the color layers at various depths gives the rainbows an attractive pastel effect.

Picture #66
3-inch-wide Fluorite Sphere-
Also called Fluorspar or Calcium fluoride
CaF2
This amazing crystal ball from China shows the typical colors of fluorite- clear, purple, green and a little blue.
The colors form in layers, stacks and pyramid patterns (phantom formations.)
This unusual stone also has vivid iris rainbow colors.
Most fluorite does not show much iris effect, unlike quartz and calcite which often, or maybe even usually, have rainbows inside the clear crystals.
Fluorite, like calcite, is a much softer stone than quartz.
It needs to be handled carefully to avoid scratching or breakage.
Both types of stone are also sun-sensitive, meaning they will fade to white or clear in strong direct sunlight.
Even sitting in a sunny window will fade out some mineral colors.
My wife calls these glittery stones “Christmas Tree Crystals,” as they remind her of the many twinkly lights on a Christmas tree.
I have yet to see another fluorite rainbow that outshines this one.

Picture #67
4-inch-wide Morganite Crystal-
“Pink Aquamarine,” Pink Beryl
Be3Al2Si6O18
Morganite crystals are considered gemstones, or precious gems, in the jewelry industry.
It is usually pink, peach, tan or orange, and occasionally occurs as rare, valuable red or lilac stones.
The mineral “beryl group” includes:
Morganite- many warm colors
Emerald- green
Aquamarine- blue or blue-green
Goshenite- colorless
Heliodor- yellow, gold or yellow-green
Bixbite- red beryl
Regardless of the color, a beryl can be very clear or completely opaque, or somewhere in-between (lightly included.)
Jewelers and gem experts always want their stones as clear as possible.
Indeed, the word “gemmy” is usually defined as totally transparent.
I prefer my stones with inclusions, as that is where the iris rainbows appear.
The beryls do not often have a lot of rainbows because the crystals tend to be much smaller than quartz crystals, or much milkier/misty as they get larger.
Any clear stone COULD have rainbows, but quartz and calcite have the most plentiful visible iris inclusions.
Note the chemical formula for beryl- lots of silicon and oxygen.
Beryl is a cousin of quartz, with aluminum and beryllium added, along with traces of manganese metal.
